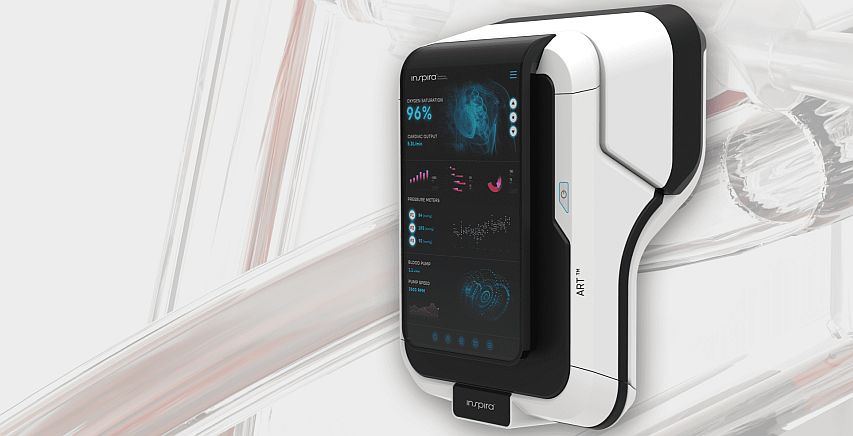
Inspira develops the “friendly” alternative for the ventilation machine
2 January, 2023
The device will allow for the oxygenation of patients’ blood while they are awake and eliminate the usage of the risky medical ventilator. "We already have hundreds of millions worth of orders"
The first days of the COVID-19 pandemic, at the start of 2020, were marked by the shortage of medical ventilators for supporting patients who lost the ability to spontaneously breathe. However, the pandemic also demonstrated the risks and the many complications involved in using these machines as the only alternative for patients who suffer from respiratory failure. The high mortality rates and the medical complications among those patients intensified the need for a replacement that will provide respiratory support in a less invasive manner while the patient is awake.
One of the main indicators leading to connecting the patient to a ventilating machine is a dangerous decrease in the level of oxygen in the blood (saturation), and the main causes might be Pneumonia, Sepsis, Corona Virus or Acute Respiratory Distress Syndrome (ARDS). Inspira Technologies from Israel is the developer of Inspira Art – a device intended for blood oxygenation safely and effectively while the patient is awake, thus eliminating the need to anesthetize the patient and connect them to a ventilator machine.
In a conversation with Techtime, Dagi Ben Noon, Inspira CEO and co-founder explains the need for such a system. “The medical team can tell, based on saturation level and the amount of oxygen flowing through the face mask, that the patient is on their way to be connected to the ventilation machine. When the patient is unconscious and on a ventilator, the team cannot communicate with them, and they are fed by measurable parameters only. In addition, a mechanical ventilator may damage the lungs and the recovery process is very long. There is a too wide ‘gap’ between oxygen face masks and ventilator machines – and our product is bridging this gap”.
Inspira’s device is designed for drawing blood through a dual-lumen cannula inserted into the jugular vein, enriching it with high concentrations of oxygen, and removing CO2. Then, the enriched blood is returned to the body until the saturation level is balanced. The process may last for several hours and is carried out when the patient is awake. By drawing smaller volumes of blood, the system ensures minimal risk for blood coagulation, hemolysis, bleeding, and infections.
The sensor that turned into a product
Inspira is planning on initiating a clinical trial for its ART device during 2024, and subsequently, filing a De Novo Classification request from the FDA. “We have already received hundreds of millions of dollars worth of orders through distributors, which reflects the market’s anticipation for this kind of solution. We are also communicating with the medical equipment giants and they also expressed interest. Our device is relevant for a large number of patients arriving at the hospital with respiratory failure”. In July 2021, Inspira was issued on Nasdaq, and since its establishment, the company has raised about $37 million. The company employs around 40 employees and is currently in the middle of another recruitment process.
One of the most significant components within the ART system is an optical sensor developed by the company, which allows for measuring, contactless, the levels of gas in the blood and estimating potential damage to the blood cells during drawing. Currently, in order to measure these parameters, frequent invasive blood tests are needed. Inspira’s sensor provides the ability to continuously measure in a non-invasive fashion. Using machine-learning algorithms, the sensor analyzes light reflections from the blood and gets information regarding parameters such as partial oxygen and CO2 pressures, and levels of saturation, hemoglobin, and hematocrit.
The sensor, developed as a component within the ART system, emerged as an independent product with various usages. It could be used in many procedures where blood oxygenation is required, such as during open-heart surgery. As a result, the company is now developing the sensor as a product named HYLA. Last month, the company announced signing an agreement with Sheba hospital for performing clinical trials on the new product. The trials, expected in Q1 2023, will examine HYLA during open-heart surgery where a Cardiopulmonary bypass machine is being used.
“Originally, the HYLA sensor was developed for the Inspira ART system, in order to improve safety when using it. However, we soon realized we had a unique solution that might be used for other medical systems. This is a huge market, and we have already signed agreements with distributors. The development is expected to be completed by the end of 2023, and the next step is the regulatory approval”.
Posted in: ElectroOptics , Medical , News
Posted in tags: Inspira