Check-Cap Reveals the Secrets of its X-ray Capsule
29 April, 2019
About 12 mm diameter and 34 mm length, C-Scan capsule generates a perfect image of the colon's inside walls, while passing naturally

Check-Cap from Osfiya in the Mount Carmel, Israel, is approaching a major Milestone: soon it will finalize Post-CE and Pilot clinical trials needed to start a pivotal trial in the US to receive FDA Marketing Approval for its revolutionary X-ray ingestible imaging capsule system for colorectal cancer screening. Soon after that, it will enter the phase of massive marketing and high volume production. Starting with a “soft launch” Israel and Europe, where has is already approved for marketing, and then to enter the lucrative US market in 2021.
Check-Cap was founded in 2005 by Dr. Yoav Kimchi, after a family relative of him was diagnosed with colorectal cancer. He sought a solution to the biggest hindrance in colorectal cancer: Low readiness of men and women over the age of 50 (population at risk) to do colonoscopy. It is an unpleasant screening procedure, yet highly advised by experts to increase survival rates, but only 60% of the target population in the US do go through the colonoscopy test.
Dense Suite of Technologies
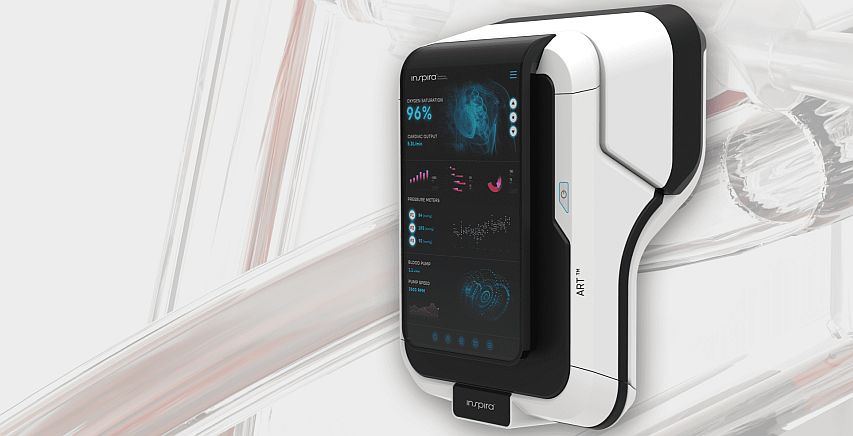
To answer the the problem, Check-Cap developed a complex miniature X-ray capsule, C-Scan. Utilizing ultra-low dose X-ray and wireless communication technologies, it generates information on the contours of the inside of the colon as it passes naturally. Thus, provides a preparation-free, colorectal cancer screening method. C-Scan is about 12 mm diameter and 34 mm length. It includes a rotating X-ray source, surrounded by six detectors, a bi-directional RF communication, a safety mechanism to contain the radiation in emergency cases, power management subsystem and unique analytics and modelling algorithm.
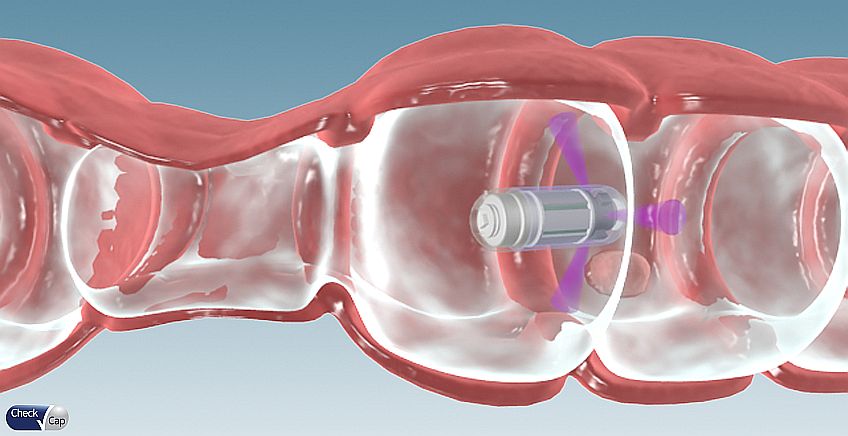
In a standard X-ray device, the x-rays emitted from the source penetrate through the body and are absorbed by a detector on the other side. Check-Cap’s detectors are different: they measure the back-scattered photons bouncing back from the intestinal tissue. Since the photons released by the interaction of the X-ray radiation with the share the same amount of energy, it utilizes a photon counter to get the data needed to build the computerized model of the tissue. The photons scattered from the intestinal content have a different amount of energy. The result is a clean image with perfect distinction between content and tissue, eliminating the unpleasant process of bowel cleansing required in a standard colonoscopy.
X-ray testing on the Road
The capsule’s data and location are recorded by 3-axis sensor located on the patient’s back. According to Check-Cap’s CEO, Alex Ovadia (photo above) the screening process does not interfere with the with patient’s daily routine. “A very complex algorithm synchronize between the tracking device and the capsule inside the bowel. During the process, the patient can drive his car, and use electronic devices and move around. We identify the entry of the capsule into the colon, and by using learning algorithm, we can improve the results without having to repeat the test on the patient.”
Ovadia’s mission today is to bring the technology into the market. Previously a defense engineer working on Elbit’S sophisticated Helmet Mounted Systems (used by F-35 fighter pilots) and GM of Philips CT division, he joined the company a year ago to lead the next phase: “Adapting the test as much as possible to home care environment. Today we are re-shaping the X-ray source and moving from Tungsten isotope to Osmium isotope. Osmium life cycle is almost as long as the screening process itself, freeing the service provider from complicated evacuation process.”
The company also redesign the tracking device to make it much smaller and lighter and has entered into a development project of cloud based analytics platform. “The patient will be able to download the data through wireless connection, from the system directly into the cloud with no need to visit a clinic.” The potential market is huge. The company estimates that the Toal Available Market in the US alone consists of 89 million people. In Europe the willingness to do the screening test is even lower: C-Scan will address 220 million people in Europe and approximately 348 million in China.
Posted in: Medical , News , Technology
Posted in tags: Check-Cap