Medtech company Check-Cap had completed in the last year foundation of an independent production line for serial manufacturing and the assembly of its X-ray capsule, intended for detecting pre-cancerous polyps in the colon. The new facility, located in Isfiya and spreads over 550 meters, is designed for producing the number of kits required for the 1000-patients pivotal study currently carried out in the U.S. According to the company, the line is expected to expand in order to support the company’s needs at the early stages of the commercialization phase, following the study conclusion and FDA approval.
In a conversation with Techtime, CEO Alex Ovadia says: “The complete assembly of the system is carried out here, in Israel. With a large company foresight, we have established the foundation for moving towards the commercialization phase. We are long-distance runners; we plan for years ahead”.
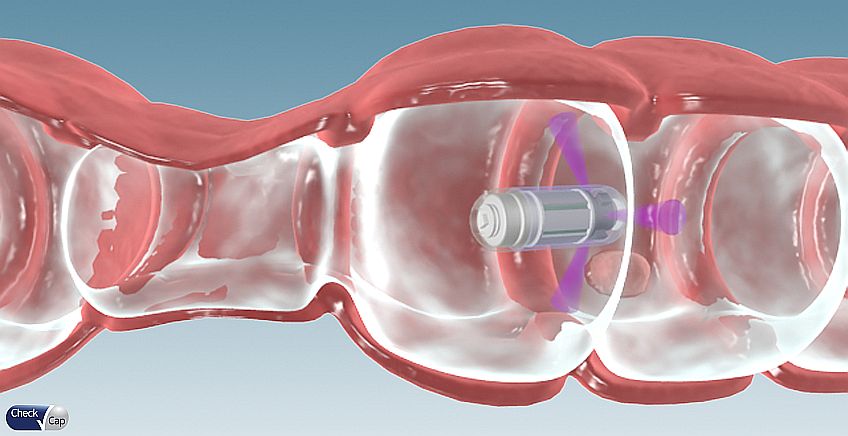
The X-Ray source, which is the isotope inside the capsule that emits the X radiation during the process, is also manufactured in Israel, in a designated production line established by the company in Petach-Tikva.
The crucial clinical study has started
In May 2022, Check-Cap announced the initiation of the pivotal clinical study, conducted in Israel and the U.S. The study was postponed by over a year as a result of the COVID-19 crisis and various logistic issues.
The study will include approximately 1,000 subjects ages 50-75 and is composed of two phases; the first phase, already started and expected to end in the middle of 2023, is meant for further calibration of C-Scan for the average risk U.S. population and will enroll up to 200 subjects. The second phase consists of approximately 800 subjects, half of them in Israel, and will continue for about a year. In this phase, the performance of the system will be compared to a traditional colonoscopy.
Sensitivity, prevalence, and adherence
In the field of colorectal cancer detection, there are currently several accepted methods. Some are intended for detecting polyps, such as the traditional colonoscopy and the PillCam camera of the Israeli Given Imaging Company (currently owned by Medtronic). These tests both require preparation and cleaning of the colon in advance. On the other hand, there are tests intended for diagnosing findings that may indicate a cancerous state. Among these Fecal Immunochemical Test (FIT), Cologuard from Exact Sciences Company, based on detecting cancerous DNA in the stool, and Liquid Biopsy tests intended for detecting cancerous biological markers in the colon. Check-Cap’s capsule is designed to detect pre-cancerous polyps in the colon without cleaning the colon before the test.
The efficiency of a test is derived from three aspects: Sensitivity – the accuracy level of the test in detecting the presence or absence of suspicious findings. Prevalence – the frequency of the finding which is in the focus of this test. For instance, a liquid biopsy test is intended to detect biological markers that may indicate the presence of colorectal cancer, a finding whose prevalence in the population is relatively low (about 0.5% of the population at average risk). On the other hand, Check-Cap’s test and traditional Colonoscopy are intended for detecting pre-cancerous polyps – a finding whose prevalence in the population is about 25%, which is considerably greater.
The third aspect, which is one of the most crucial in the field of early colorectal cancer detection is adherence – the willingness of the patient to take the test. In fact, this is the problem Check-Cap is striving to address: the low adherence among the high-risk population to take the traditional colonoscopy test, mainly because it is an unpleasant, invasive test that requires cleaning the colon in advance.
Despite the delays in the clinical study, CEO Ovadia thinks the company’s competitive advantage is preserved when compared to the alternative solutions: “Our advantage is reflected in the optimal integration of sensitivity, prevalence, and adherence. The combination of these three aspects is the determinant of the test’s “yield” – and is also the key for decreasing mortality rates from colorectal cancer. In this manner, the advantage of our capsule, even when compared to colonoscopy, is in the extensive willingness to take it.
A friendly alternative to colonoscopy
Check-Cap has developed a user-friendly solution for detecting pre-cancerous polyps in the colon. The system is composed of an ingested capsule containing an X-Ray source and a control system attached to the back of the patient, which positions and manages the tests. In addition, the system includes software for processing the data and provides a 2D & 3D mapping of the colon’s interior wall.
Unlike colonoscopy, this test is a non-invasive, prep-free test. In fact, the patient swallows the capsule and continues their daily routine with no discomfort. At the end of the process, the capsule is naturally emitted from the body.